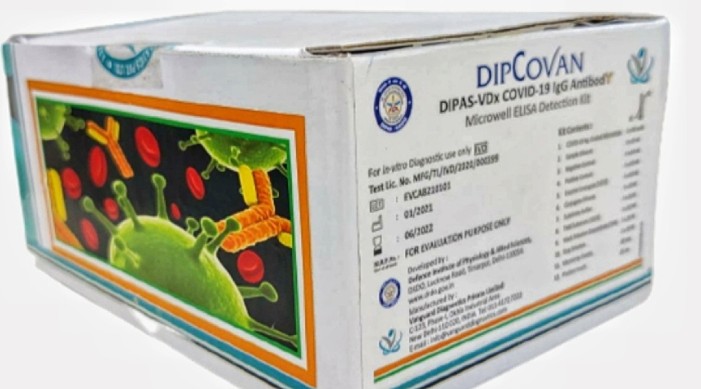
New Delhi, May 21 (INN): A laboratory that is part of India's Defence Research and Development Organisation (DRDO) has developed a Covid-19 antibody detection kit 'DIPCOVAN'. The DIPAS-VDx COVID-19 IgG Antibody Microwell ELISA for sero-surveillance has been developed by the Defence Institute of Physiology and Allied Sciences (DIPAS). Providing more information, the Ministry of Defence on Friday said the DIPCOVAN kit can detect both spike as well as nucleocapsid (S&N) proteins of SARS-CoV-2 virus with a high sensitivity of 97 per cent and specificity of 99 per cent. The kit has been developed in association with DRDO's industry partner Vanguard Diagnostics Pvt Ltd, a development and manufacturing diagnostics company based at New Delhi. The DIPCOVAN kit was developed indigenously by the scientists, followed by extensive validation on more than 1,000 patient samples at various COVID designated hospitals in Delhi. Three batches of the product were validated during the last one year. In April this year, the antibody detection kit was approved by the Indian Council of Medical Research (ICMR). A month later, the product received the regulatory approval from the Drugs Controller General of India (DCGI), Central Drugs Standard Control Organisation (CDSCO), Ministry of Health and Family Welfare, to manufacture for sale and distribution. DIPCOVAN is intended for the qualitative detection of IgG antibodies in human serum or plasma, targeting SARS-CoV-2 related antigens. "It offers a significantly faster turn-around-time as it requires just 75 minutes to conduct the test without any cross reactivity with other diseases," the defence ministry said. The kit, with a shelf life of 18 months, will be launched commercially in the first week of June and is expected to be available at about Rs 75 per test. The kit will be very useful for understanding COVID‐19 epidemiology and assessing an individual's previous SARS‐CoV‐2 exposure, the defence ministry pointed out.